Capturing frames of photosynthesis and other molecular gymnastics in action means reaching a shutter speed that makes fast look very, very slow — so fast that physicists are just now working their way up to it.
Therein lies another problem: Even when they manage it, they might not know it. Physicists can’t exactly eyeball the molecules in motion and compare what they capture with what they see, as they could with a digital photo of a macro-level scene. Such is life when studying molecules that morph and snap and spin on timeframes so short they make seconds seem like billions of years.
But the University of Nebraska–Lincoln’s Colton Fruhling and colleagues at the Extreme Light Laboratory have proposed a solution to the second problem that could prove vital whenever their fellow physicists manage to fully resolve the first.
The first usually involves firing bunches of electrons at molecules — often while blitzing the molecules with a laser to stimulate a photochemical reaction — then measuring the ways those electrons diffract from the molecules. Along with heaping helpings of theory and math, those diffraction patterns can help discern the positions of the atoms and the lengths of the bonds that make up the molecules, essentially capturing frames of a photochemical reaction that can be stitched together into a pseudo-film.
The duration of a corresponding electron bunch basically acts as the laser-physics equivalent of shutter speed. Just as with a digital camera, that shutter speed needs to at least match the speed of a subject in order to capture it with any real fidelity. And knowing that shutter speed is essential to confirming the legitimacy of the resulting frames.
That turns out to be difficult when the chemical reactions of interest occur in mere femtoseconds or even attoseconds. One femtosecond compares to one second as one second compares to about 31 million years; for an attosecond, it’s about 31 billion years, or roughly twice the estimated age of the universe.
Physicists have successfully concocted methods for measuring the duration of electron bunches that last just several femtoseconds, but not attoseconds — the blink-and-and-you’ve-missed-it-10-billion-times speed at which many chemical reactions occur.
“So you need to have a way to measure that you are (operating in) attoseconds,” said Fruhling, a doctoral candidate on track to graduate by spring 2021. “You can see how fast a camera shutter moves, because you’re watching it. Our eyes are fast enough for that. But you can’t see an attosecond.
“People want these attosecond electron beam sources, but they also need to make sure that they’re characterizing them and making sure that they’re actually attosecond, so we can believe the science that comes out of that.”
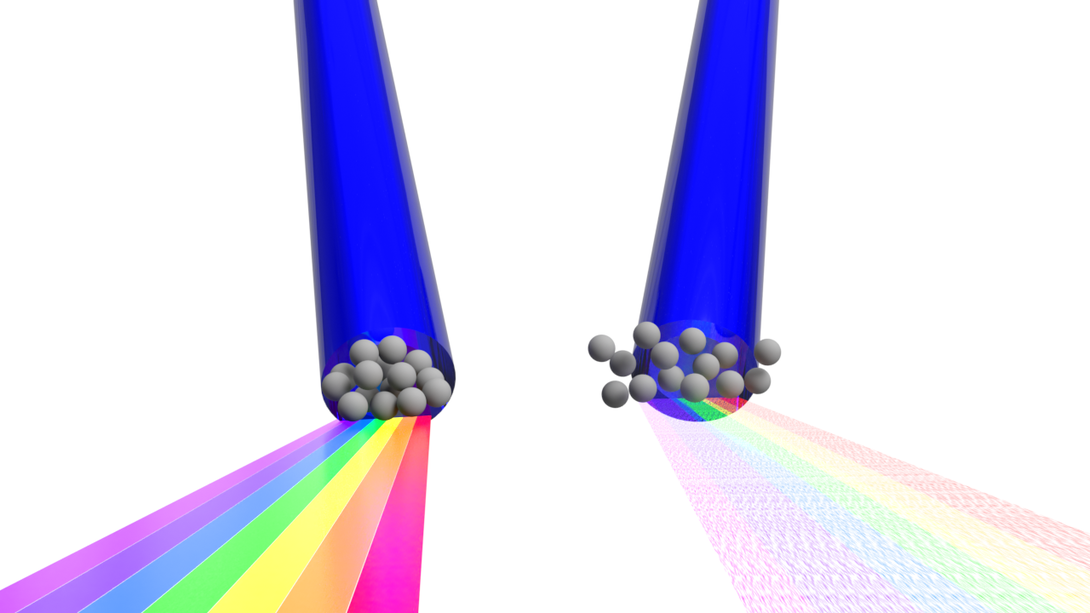
Fruhling eventually recognized a potential solution in the form of Thomson scattering, a phenomenon that the Extreme Light Laboratory has studied for years. In the linear version of the phenomenon, a laser-struck electron ultimately emits light at the same frequency, or color, as the laser itself. In the nonlinear version, the laser is intense enough that the electron begins oscillating in complex trajectories at close to the speed of light. That drives the electron to emit not just the original color but multiple wavelengths, or broadband radiation.
Fruhling was coding a model to simulate that nonlinear version when he began thinking about how he might use it. He knew that some methods used to measure femtosecond bunches rely on the fact that another measurable property of wavelengths — coherence — will change according to the size of the electron bunch itself.
Coherence basically describes the extent to which the frequency, shape and other signature traits of waves sync up with one another. It’s coherence that results in the focused, narrow beam of a laser and distinguishes it from the incoherent wavelengths of other light sources. And it so happens that wavelengths longer than an electron bunch will emit coherently, similar to a laser, while those shorter than the bunch will emit incoherently.
Determining the size of the electron bunch — and by association, its duration, or shutter speed — then becomes a matter of identifying the size threshold that separates the coherent and incoherent waves of light. Unfortunately, linear Thomson scattering doesn’t yield the right range of frequencies for measuring the ultra-short yet moderate-speed electron bunches that are needed to investigate attosecond reactions.
But if Fruhling’s model is correct, the nonlinear, broadband scattering — the kind that can be generated by an ultra-intense, precisely calibrated laser — does produce frequencies in that range. And if so, he said, that would make it uniquely suited to measuring the duration of attosecond bunches.
“This is the only method I know of that can do this,” said Fruhling, who reported the conclusion with Donald Umstadter and Grigory Golovin in the journal Physical Review Accelerators and Beams.
Fruhling didn’t come by the milestone easily, devoting more than three years to writing code that can model the trajectory and coherency effects of every electron within, say, a 5,000-electron bunch — a level of specificity unmatched by any counterpart he’s come across. He also ended up translating the code across three programming languages while refining the interface to make it usable across as wide a range of conditions as possible.
Now he just has to wait for fellow physicists to test his claim in the lab, and hopefully verify it, by actually producing electron beams that last for just attoseconds.
“I can’t toot my own horn until it’s done experimentally,” Fruhling said. “But I think it could be very useful.”







