Pinning DNA-sized ribbons of carbon to a gas sensor can boost its sensitivity far better than any other known carbon material, says a new study from the University of Nebraska-Lincoln.
The team developed a new form of nano-ribbon made from graphene, a 2-D honeycomb of carbon atoms. When the researchers integrated a film of the nano-ribbons into the circuitry of a gas sensor, it responded about 100 times more sensitively to molecules than did sensors featuring even the best-performing carbon-based materials.
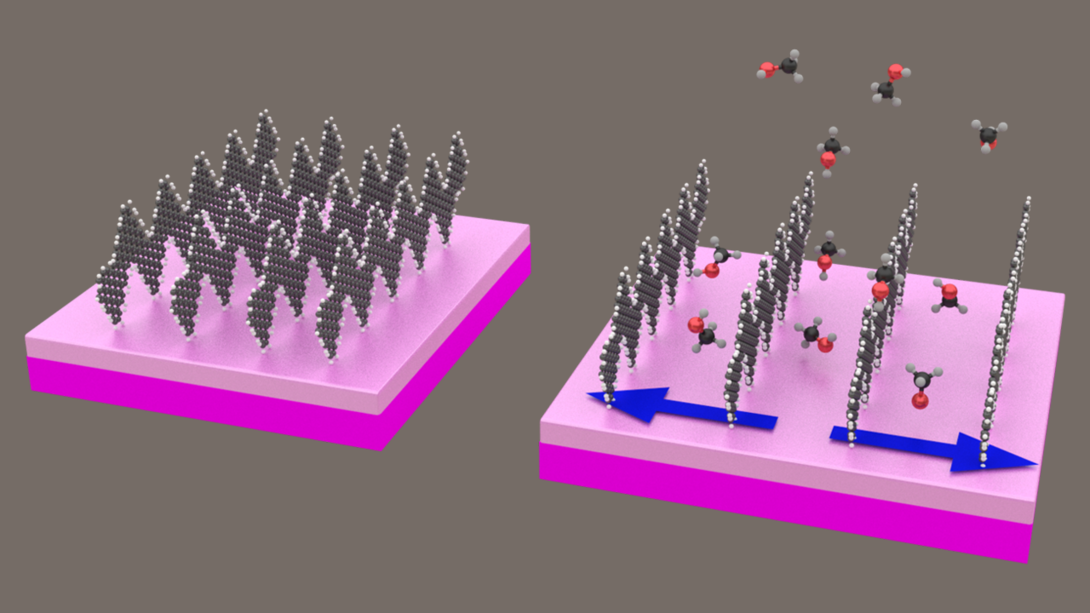
Reporting their findings in the journal Nature Communications, the researchers showed that gas molecules can dramatically alter the electrical resistance of nano-ribbon films. Different gases produced varying resistance signatures, allowing the sensor to distinguish among them.
“With multiple sensors on a chip, we were able to demonstrate that we can differentiate between molecules that have nearly the same chemical nature,” said Sinitskii, a member of the Nebraska Center for Materials and Nanoscience. “For example, we can tell methanol and ethanol apart. So these sensors based on graphene nano-ribbons can be not only sensitive but also selective.”
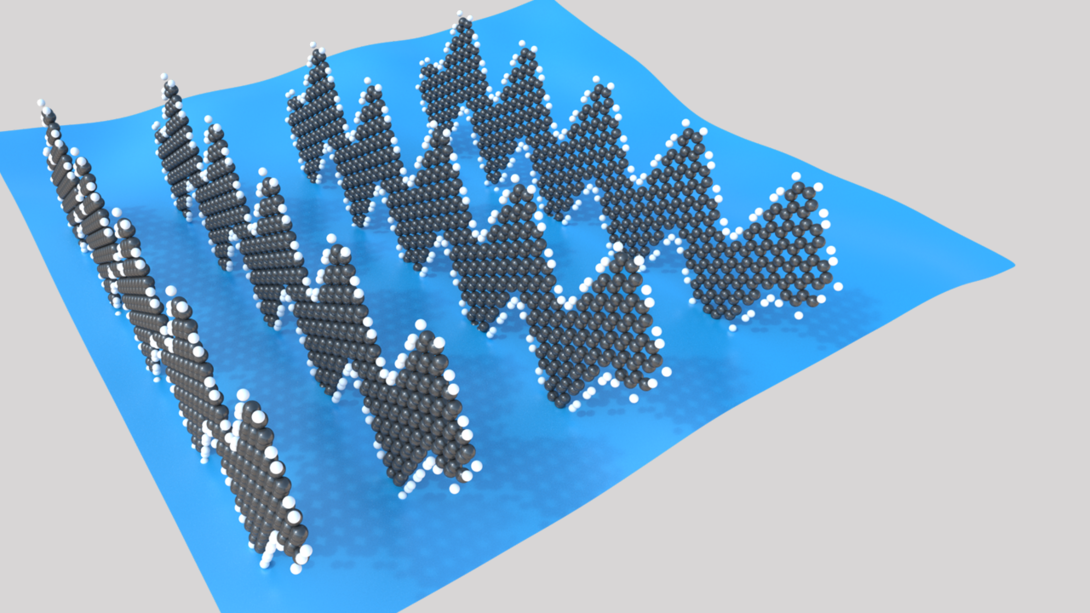
Sinitskii and his colleagues suspect that the nano-ribbons’ remarkable performance stems partly from an unusual interaction between the ribbons and gas molecules. Unlike its predecessors, the team’s nano-ribbons — which resemble ordered rows of Charlie Brown’s shirt stripes — stand vertically rather than lying flat on a surface. The team has proposed that gas molecules can nudge these rows apart, effectively lengthening the gaps between nano-ribbons that electrons must jump to conduct electricity.
Enter the (benzene) ring
Graphene, whose 2004 discovery eventually earned a Nobel Prize, boasts unmatched electrical conductivity. But the material’s lack of a band gap — which requires electrons to gain energy before jumping from their near orbits around atoms to an outer “conduction band” that drives conductivity — initially prevented researchers from switching off that conductivity. This, in turn, posed challenges to applying graphene in electronics that require adjusting the material’s conductivity at will.
One potential solution involved trimming sheets of graphene down to nanoscopic ribbons that computer simulations suggested would possess the elusive band gap. This proved difficult to do with the atomic precision needed to preserve the properties that made graphene appealing in the first place, so researchers began fabricating ribbons from the bottom up by strategically snapping together molecules on certain types of solid surfaces. Though the process worked – and the resulting ribbons did have a band gap – it limited researchers to fabricating just a few ribbons at a time.
In 2014, Sinitskii pioneered an approach that could mass-produce nano-ribbons in a liquid solution, a vital step toward scaling up the technology for electronic applications. But the films made from these nano-ribbons were not conductive enough to perform electrical measurements. The team’s newest study adapted the original chemical approach by adding benzene rings — circular molecules with six atoms of both carbon and hydrogen — onto either side of a first-generation nano-ribbon. These rings widened the ribbon, reducing its band gap and enhancing its ability to conduct electricity.
“People do not often think of graphene nano-ribbons as a sensor material,” Sinitskii said. “However, the same (property) that makes the nano-ribbons good for devices such as transistors – the ability to change their conductivity by several orders of magnitude — is also what makes them good for sensors.
“It is possible to design many different kinds of graphene nano-ribbons with very diverse properties. Only a few types have been experimentally demonstrated so far, but there are many interesting theoretical predications about ribbons that are yet to be synthesized by chemists. So it is very likely that new nano-ribbons with even better sensor characteristics or other exciting properties will be developed in the near future.”
Sinitskii authored the study with Nebraska’s Alexey Lipatov, research assistant professor of chemistry; Mohammad Mehdi Pour and Mikhail Shekhirev, doctoral students in chemistry; Rafal Korlacki, research engineer in electrical and computer engineering; the University of Illinois at Urbana-Champaign’s Adrian Radocea, Ximeng Liu, Tao Sun, Narayana Aluru and Joseph Lyding; and Victor Sysoev and Andrey Lashkov of Saratov State Technical University.
The researchers received support primarily from the National Science Foundation, the Office of Naval Research and the Nebraska Research Initiative.







