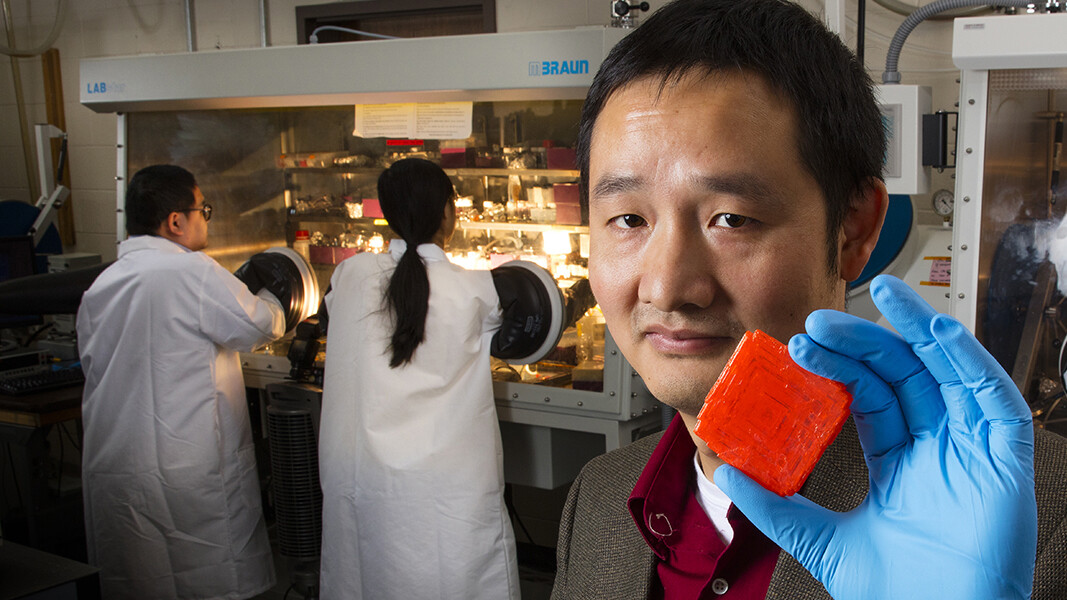
Sticks and stones may break bones, but a research group led by the University of Nebraska-Lincoln has set out to ensure that the ensuing X-ray will never hurt you.
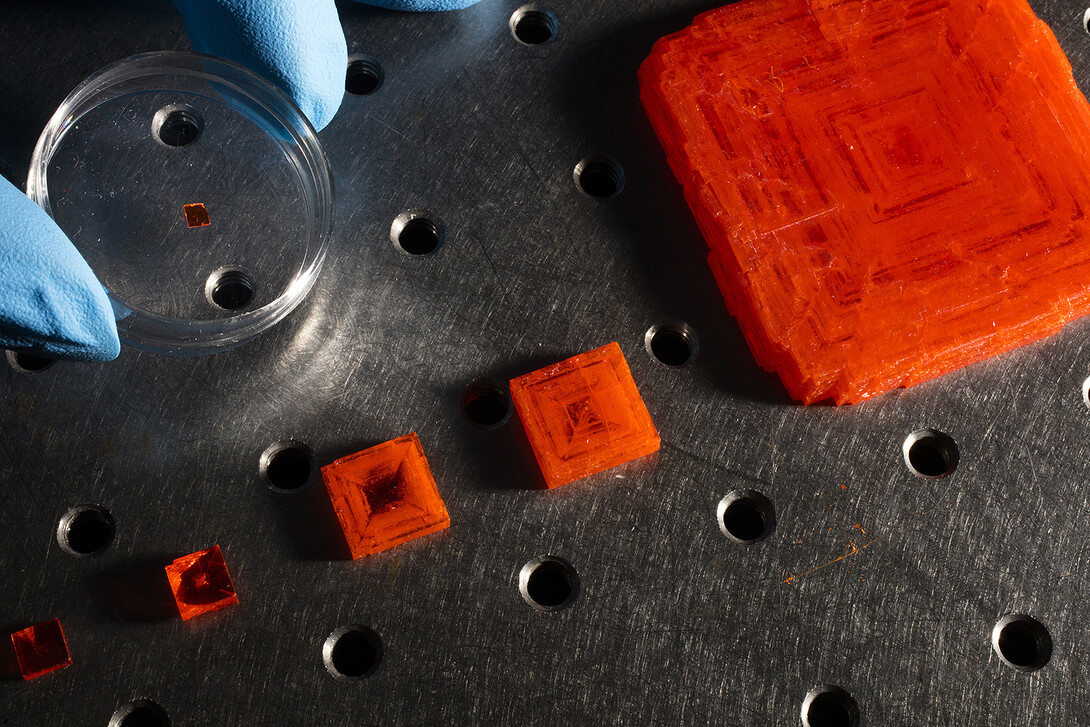
In a March 21 study published by the journal Nature Photonics, the researchers reveal a crystalline material that is four times more sensitive to X-rays than leading commercial detectors. Known as methylammonium lead tribromide, the material can detect an X-ray dose about 11 times lower than that required for many medical applications.
X-rays have become a staple of medical diagnoses since their discovery in 1895, helping detect fractures in bones and reveal tumors in tissue. The rise of global terrorism has also increased their use for security purposes, particularly in the transportation industry.
Yet X-rays also represent a form of radiation that can damage tissue and raise the lifetime risk of developing cancer, which increases with the dose and number of exposures. That risk rises further among those exposed to X-rays at a young age, according to the National Institute of Biomedical Imaging and Bioengineering.
UNL engineer Jinsong Huang and colleagues recently began exploring methylammonium lead tribromide as a candidate for limiting this exposure. The material belongs to a family of compounds known as perovskites that Huang has studied since 2013 in his efforts to improve the performance of solar cells and photodetectors.
“If you look at the history of X-ray detectors, the materials used for them are usually also good for photovoltaic devices,” said Huang, a Susan J. Rosowski associate professor of mechanical and materials engineering. “This material is almost perfect for X-ray applications.”
The material’s X-ray sensitivity stems in part from its large atomic weight, or the number of protons residing in each of its atoms. These heavier atoms are able to absorb more of the high-energy photon particles that constitute X-rays, making their parent material sensitive to smaller doses of the radiation.
Certain detectors take advantage of the fact that X-ray photons possess enough energy to knock loose atom-orbiting electrons, which have a negative charge and leave positively charged “holes” in their wake. When electrodes are placed at either end of a semiconducting material such as methylammonium lead tribromide, the holes and electrons migrate in opposite directions to produce pulses of electric current that are ultimately translated into digital images.
Because methylammonium lead tribromide naturally allows positive and negative charges to flow through it at breakneck speeds and for long distances, it could prove ideal for the so-called direct flat panel detectors that employ this very principle, Huang said.
“So we basically need to stop the X-ray, convert its energy into electron-hole pairs, and then extract these charges to electrodes or circuits at a high yield that approaches 100 percent,” he said. “The sensitivity of the detector is defined (largely) by this efficiency. If we know the X-ray intensity coming into the crystal, then we can estimate how much current we can get out of it.”
The Nature Photonics study reports that these properties could make methylammonium lead tribromide a substantial upgrade over amorphous selenium, a material that Huang called a “workhorse” of the X-ray detector industry.
“Amorphous selenium is not a great material,” Huang said. “Everyone knows that. But it’s still popular in this application simply because it’s relatively simple to make, and you need a large array.
“To compare with amorphous selenium, the perovskite needs to be equally simple to make. Now we are close to that. We have demonstrated that we can make a very large array of perovskite crystals. I don’t see many intrinsic barriers to making this on the order of amorphous selenium.”
Huang co-authored the study with UNL postdoctoral researchers Haotong Wei and Yanjun Fang, along with researchers from Ohio State University, the University of Groningen (Netherlands) and the University of Rochester.
The group’s work received funding from the Defense Threat Reduction Agency, the European Research Council and the National Science Foundation.

Share
News Release Contact(s)
Related Links
Tags
High Resolution Photos

HIGH RESOLUTION PHOTOS
HIGH RESOLUTION PHOTOS








