· 4 min read
Research Roundup | Sept. 21
Welcome to the Research Roundup: a collection of highlights from the latest Husker research and creative activity.
‘One of our major pandemic projects’
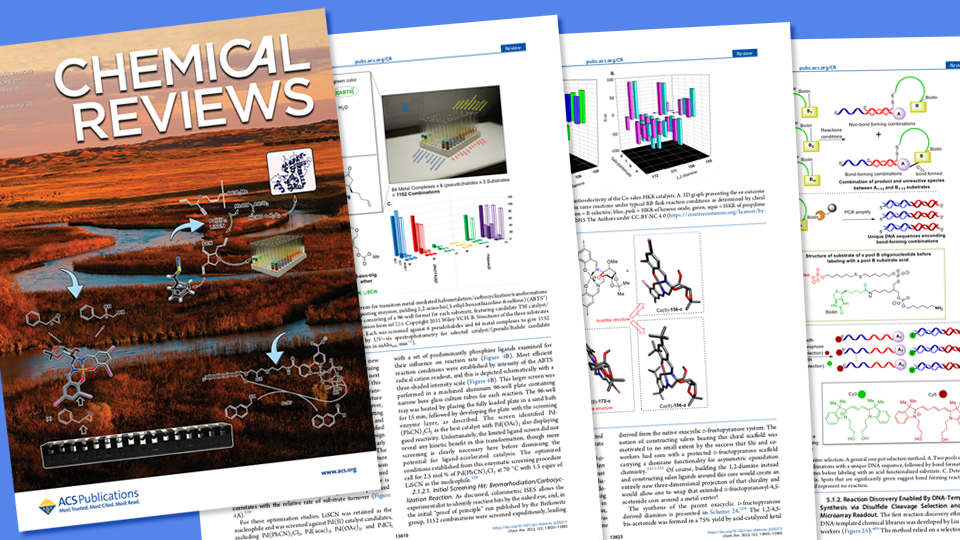
An 81-page, Husker-authored overview of how large biological molecules have stimulated the discovery of innovation-driving catalysts and chemical reactions recently graced the cover of Chemical Reviews, a journal published by the American Chemical Society.
Coming from the lab of David Berkowitz, Willa Cather Professor of chemistry at Nebraska, the article reviews the ways that three classes of biomolecule — enzymes, antibodies and nucleic acids — have streamlined the hunt for catalysts and the vital chemical reactions they spur.
“This review might be regarded as one of our major pandemic projects,” said Berkowitz, who authored the paper with doctoral alumni Stephany Ramos De Dios and Christopher McCune, postdoctoral researcher Virendra Tiwari, and former Nebraska postdoc Ranjeet Dhokale. “The period of brainstorming, outlining the approach, researching the literature, writing and polishing overlapped very closely with the critical two years of the pandemic, and proved to be a really good use of our time in this period where sometimes it was difficult or even impossible to do research in the laboratory.”
Chemical reactions — rearrangements of the atoms in elements or compounds to yield other chemicals — are central to building replicas or variations of carbon-based molecules otherwise produced in nature. Those lab-synthesized molecules have found wide use in developing pharmaceutical drugs, studying biochemical processes and designing advanced materials.
The chemical reactions that ultimately yield those molecules often rely on catalysts, or substances that accelerate the reactions without undergoing any change themselves. In their review, Berkowitz and his colleagues outline many biomolecule-based methods that have been devised to sense and read out the properties of those essential catalysts. One of those methods, In Situ Enzymatic Screening, was conceived by the Berkowitz group to rapidly explore reactivity space and evaluate catalyst candidates. With support from the National Science Foundation, those efforts have since propelled the discovery of reactions and ultra-tailored chemistry with particular promise for fighting disease.
Though supercomputing and machine learning have opened up new, computation-intensive avenues of discovery, Berkowitz said the review underscores the continued importance of experimental approaches.
“Reaction discovery and catalyst optimization are highly empirical endeavors,” he said, “and new tools that facilitate these sorts of experimentation endeavors are invaluable in chemistry, both in academia and in industry.”
Something’s fishy
Antibodies, which are typically produced by the immune system to combat harmful infections, can also drive allergic reactions by attacking otherwise harmless substances: pollen, peanuts and fish, among others. Though multiple test kits use antibodies to detect the presence of fish allergens, many of the commercially available versions have focused on species found mostly in the Northern Hemisphere.
Alongside colleagues from the University of New South Wales, Nebraska’s Stephen Taylor and Joseph Baumert have unveiled a test that can detect a wider array of allergy-causing fish species native to the Southern Hemisphere. A prior study of three commercially available allergen-detecting kits found that just 12 of 57 fish species were identified by all of those kits. As detailed in the journal Food Chemistry, the new test managed to detect 28 of 37 species that are regularly fished and consumed in Australia. It also identified allergy-causing fish residues in five highly processed products, including fish extracts.
Heavy metal
Huskers Stephen Morin, Jessica Wagner and Jared Fletcher have developed a simple, scalable and inexpensive method of adhering certain metals to commercially viable plastics — an appealing option for engineers of wearable electronics, smart packaging and other technologies.
Depositing functional metals on plastics has proven a useful means of adding circuitry to pliable, rounded surfaces, opening up electronic functionality to products that traditionally lack it. But the processes for depositing those metals have generally involved multiple, costly steps or yielded a weak adhesion between metal and plastic.
After treating multiple plastics with ultraviolet ozone and a class of molecules that bind especially well to charged metal atoms, the Nebraska researchers demonstrated that the treated surfaces would adhere strongly to traces of copper, nickel, silver and gold. The team then demonstrated the functionality of those traces by creating circuits that illuminated LEDs and the electrodes of a simple battery cell that it powered on and off.







