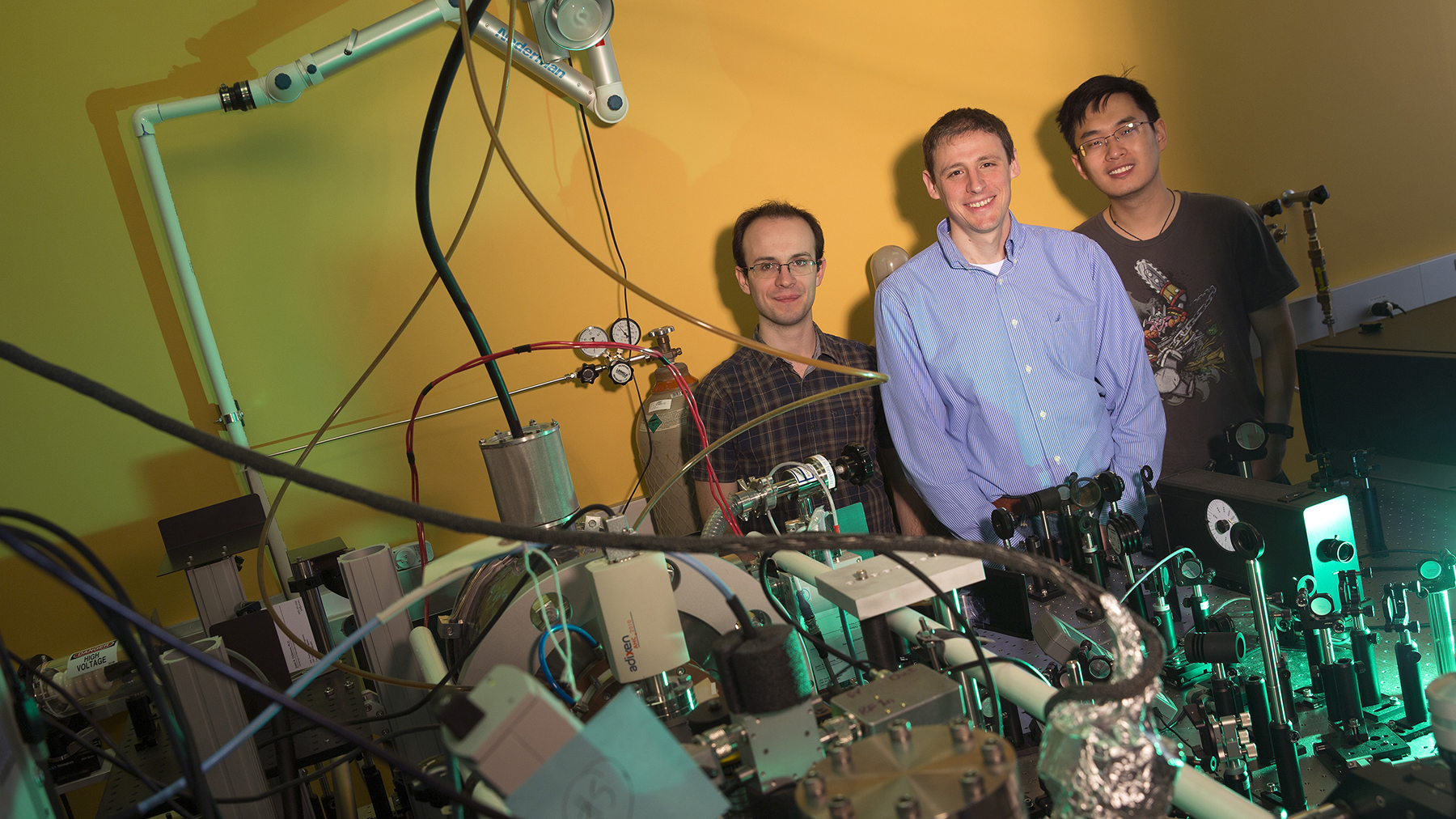
By firing laser-triggered electrons fast enough to make one second seem like 150,000 years, physicists from UNL and the SLAC National Accelerator Laboratory have advanced efforts to record the molecular reactions that drive countless chemical and biological processes.
In a study published April 5 by the journal Nature Communications, the researchers report capturing the movement of atoms on a time scale four times shorter than previously achieved. This essentially allowed the authors to record four times as many “frames” of the phenomena in action.
“This is the next big step in our quest to make movies of molecular reactions,” said co-author Martin Centurion, UNL associate professor of physics and astronomy. “We have now shown that we have achieved sufficient time resolution to capture these dynamics.”
Centurion said his team will initially use the capability to examine simple chemical reactions, which originate from the formation or breakage of bonds among atoms. He sees it proving especially useful for exploring processes that convert light into chemical energy, as occurs in photosynthesis.
“If we really see how this happens, we can maybe understand exactly what is going on,” Centurion said. “Then we can either manipulate this reaction to get (molecules) to do what we want, or we can design better ones to convert and store energy.”
The researchers accomplished their feat at the SLAC National Accelerator Laboratory, a Stanford-housed site overseen by the U.S. Department of Energy. There they employed a technique called ultrafast electron diffraction that Centurion helped pioneer in 2012.
In the new study, the method involved splitting a laser pulse into two beams. One struck nitrogen gas molecules, rotating them into periodic alignment, while the other energized a device that fired beams of electrons at those same molecules.
Shortening the duration of those electron beams allowed the research team to track atomic movement on the record-short time scale reported in the study. But doing so meant overcoming a problem: the natural tendency of electrons to repel one another, leading to longer beams and, consequently, fewer frames of the atoms in motion.
To prevent this, the SLAC accelerator made the beams “relativistic” by launching them at 99 percent the speed of light. This translated to a duration of about 200 femtoseconds – a span that compares to one second as one second compares to 158,500 years.
“So even though you have the same (repulsion) force, the effect of the force is much smaller,” Centurion said. “One way to think about this is (that) when something becomes relativistic, it’s as if it becomes heavier. Now those electrons are very heavy, so it’s the same force, but they don’t go anywhere. This way, we can get much shorter pulses on the target.”
After electrons strike and scatter from target molecules, the electrons’ waves interfere with one another to create patterns somewhat like overlapping ripples in a pond. By tracking changes in these interference patterns over time, the researchers were also able to calculate ranges for the shifting locations of the nitrogen atoms.
The researchers chose nitrogen, in part, because its atoms are the seventh-lightest among all known elements. Because lighter molecules that are excited by lasers will spin faster and align more frequently than their heavier counterparts, nitrogen served as a prime benchmark for demonstrating the technique’s improved time scale.
However, nitrogen also scatters electrons less widely than would heavier atoms, making the task of actually detecting the electron waves more difficult.
“The point was that if we could see this lighter molecule, we should be able to see heavier ones, too,” Centurion said.
But the ability to track nitrogen and other biologically important atoms should prove useful down the road, when Centurion plans to examine the mechanisms that protect organisms against the DNA-altering effects of ultraviolet light.
Based on its energy levels, UV light would be expected to damage DNA more extensively than it actually does, Centurion said. Yet DNA molecules manage to discard UV-transmitted energy as heat before it can warp the DNA’s structure. Centurion said his team may eventually subject individual DNA bases to UV light in the hope of identifying how this occurs on a molecular level.
“In terms of long-term impact, I think there are many (molecular) processes that are still not fully understood,” he said. “This could be the next step in helping to understand them.”
The Nature Communications study was authored by 23 researchers, including Jie Yang, UNL graduate student in physics and astronomy; Markus Guehr, staff scientist at Stanford’s PULSE Institute; and Xijie Wang, research technical manager at the SLAC National Accelerator Laboratory.