
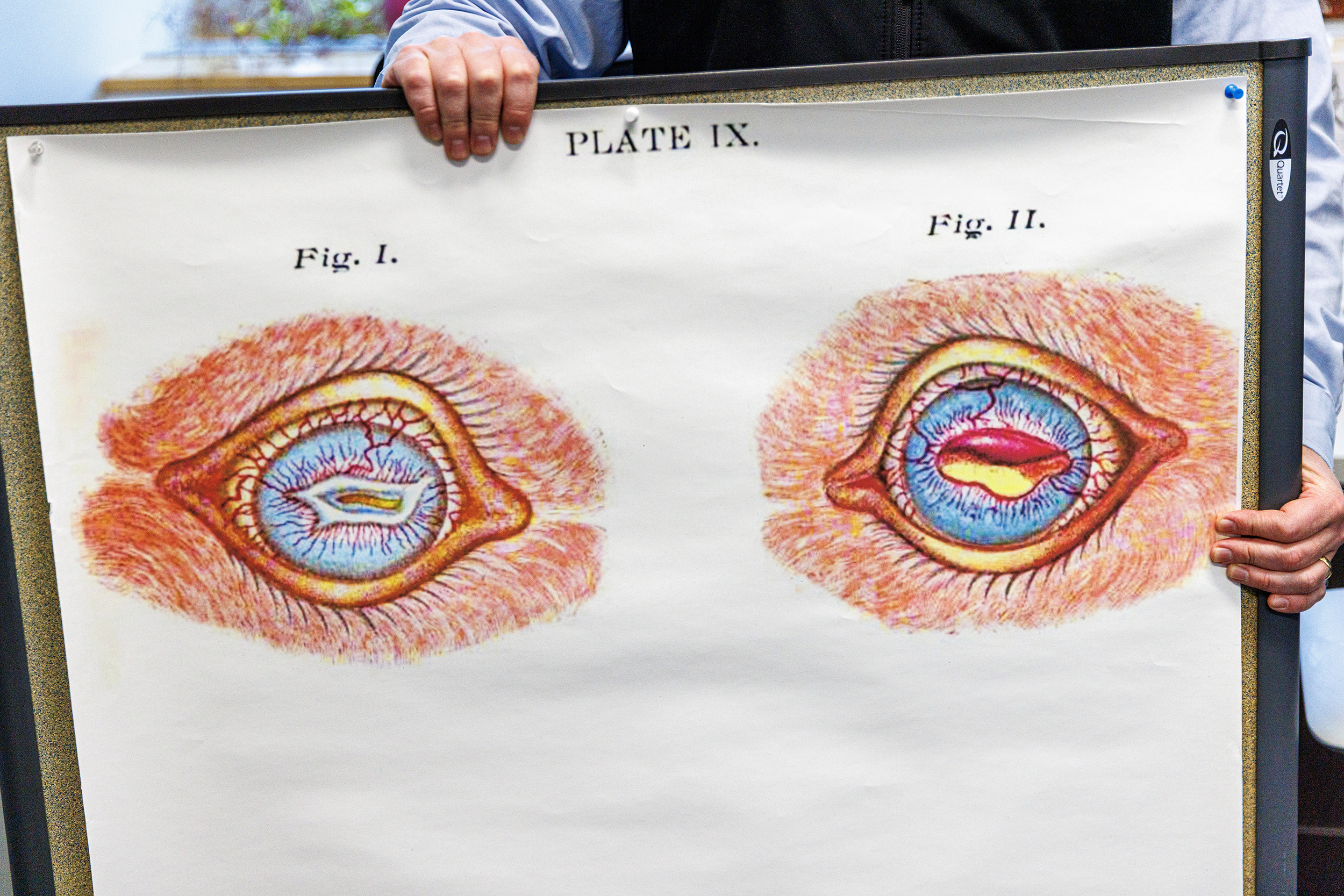
Research notes, reference materials, reports — such are the items to be found in the East Campus office of Dustin Loy, a professor in the University of Nebraska–Lincoln’s School of Veterinary Medicine and Biomedical Sciences. But Loy’s office also features an unusual drawing, a scientific one from deep in the university’s history.
The image is a drawing from one of the first known descriptions of cattle affected with pinkeye, a longtime scourge of Nebraska beef producers. The debilitating disease is primarily caused by Moraxella bovis, a tiny organism that, in the 21st century, results in annual losses to the cattle industry estimated in the hundreds of millions of dollars.
Frank Billings, a veterinary pathologist who was the first NU faculty member given a full-time research position, sketched the image of afflicted cattle for an 1889 bulletin from the Nebraska Agricultural Experiment Station. The bulletin, in which Billings described a “dense cluster of micro-organisms,” provided the first scientific description of the bacterium and the disease.
In the present day, combating bovine pinkeye — an affliction that involves a range of possible trauma to the animal’s eye — remains a major challenge for veterinary research scientists. The genetic diversity and variation of the bacterium are so complex that major gaps in scientific understanding remain, even 133 years after Billings’ initial publication. And unlike diseases such as polio and measles, which can be addressed through a vaccine, bovine pinkeye so far has remained resistant to consistently effective prevention via vaccination.
The illness — technically, infectious bovine kerato-conjunctivitis, or IBK — is the No. 1 reported disease for breeding cows and No. 2 for calves.
Two recent studies with contributions from Husker researchers are advancing scientific knowledge of the disease. A joint research project between the university and the U.S. Department of Agriculture’s Agricultural Research Service, or ARS, reported important findings about the bacterium’s genetic characteristics. And Matt Hille, an assistant professor of veterinary medicine and biomedical sciences, headed the first long-term study of bovine pinkeye vaccines, identifying shortcomings in inoculation efficacy.
Finding a solution to bovine pinkeye is hindered by the fact that a range of factors, of varying influence, contribute to the incidence of the disease.
“It’s what we call a classic disease complex,” Loy said, “with contributions from the pathogens, contributions from the host — we know that certain breeds or types of animals are more susceptible to this disease than others. And then there’s the environment, everything from dust to UV light to fly burden. All those things contribute. So there are a lot of targets.”
“A big factor, certainly, is that this is a multifactorial disease,” said Hille, whose vaccination study noted the multiple influences contributing to the disease.

The genetic complexity and variability of the M. bovis bacterium — as well as a related bacterium, M. bovoculi — at present thwart a complete understanding of the microorganisms’ ability to cause disease. Precisely what factors turn non-threatening components of the bacteria into pathogens have yet to be fully identified. Nebraska’s joint study with ARS, published in BMC Microbiology, has moved the science forward by providing significant new information on the genetic structure and diversity of M. bovis.
ARS molecular biologists Emily Wynn and Mike Clawson, at the U.S. Meat Animal Research Center in Clay Center, Nebraska, analyzed M. bovis strains provided by Loy from the university’s extensive inventory of pinkeye bacterial isolates. Loy collected the samples from veterinarians in 17 U.S. states and one Canadian province as part of his longtime work in identifying variabilities among the strains.
The ARS scientists sequenced and compared the samples’ genetic makeup, resulting in notable discoveries. The researchers identified two major M. bovis genotypes, each with different versions of a pinkeye-related toxin. They also identified certain proteins on the outer membrane of the bacterial cell.
Those findings significantly broaden scientists’ genetic understanding of the disease. This sets the stage for future research work to fill in additional gaps.
Textbooks over the decades have characterized M. bovis as readily definable and largely immutable over time, Hille said, but the ARS findings show that the reality of the microorganisms is far more complex, with ongoing changes and much more genetic information to be discovered and studied.
Understanding that genetic complexity is necessary in “getting closer to what might actually be causing the disease,” Loy said. The ARS analysis “opens a lot of avenues” for scientists to potentially identify “new virulence factors and new ways of thinking about how this might be causing disease.” Ultimately, the accumulation of that knowledge can help in developing better interventions and better targets.
The joint project is the latest in scientific collaboration by the university and ARS in recent years on cattle-related diseases.

In an academic paper published this year in the journal Vaccines, Hille described a five-year study he headed that compared pinkeye vaccine effectiveness for three study groups of calves. One group received an autogenous vaccine, developed from bacterial strains isolated from the study herd. Another group received a commonly used vaccine developed by a pharmaceutical company. A third group received a formulation consisting only of adjuvant, a component normally added to a vaccine to bolster the immune response.
The autogenous vaccine produced a significantly stronger antibody response than the other two formulations, but the effect wasn’t significant in terms of actually preventing the disease. The study involved an average of 240 calves a year at Nebraska Extension’s Eastern Nebraska Research, Extension and Education Center near Mead. Hille’s five-year project advanced the science in several ways, Loy said. It was the first vaccine study using real-world field conditions over that duration of time and that used antigens from three different species of bacteria associated with pinkeye. It stands out, too, because Hille, in collaboration with virologist Hiep Vu of Nebraska’s Department of Animal Science, developed an analytical approach examining the animals’ immune responses and whether they came down with the disease. The result was “a unique and powerful study to at least show where our gaps are in the vaccines.”
Hille’s study included analysis of calves’ antibody response to a particular biological component — type IV pilus protein — long associated with the M. bovis bacterium. The vaccines’ failure to prevent the disease, despite the animals’ immune responses to the protein, squares with the genetic findings by ARS scientists indicating that prevention is unlikely to be consistently effective until more of the bacterium’s genetic composition and contribution to disease is understood, and appropriate changes are made to current vaccine formulations.
Other contributors to the project were Matthew Spangler, with the Department of Animal Science, and Kelly Heath, with the School of Veterinary Medicine and Biomedical Sciences.
Disease prevention strategies have essentially been unchanged since the 1960s, Loy said, and need updating. “We’re getting closer with this information,” he said, “to really provide some background research that can help move that forward.” Scientific struggles with vaccination complications extend back to the 1880s, at the time Billings was laying the foundation for animal biological study at the university.
Billings “was a pathologist looking at tissues and really trying to understand disease,” Loy said. “He really took a crack at bovine pinkeye and tried to apply the new concept of germ theory.”
The university, along with ARS, continued the study of the disease in the 20th century. George Washington Pugh Jr., the first Black scientist with ARS, made major advances in understanding the role of M. bovis in pinkeye. In 1987, Pugh was a co-author of an academic paper on the disease with Douglas Rogers — a veterinary pathologist who, in this century, was a mentor to Loy on East Campus.
For the 1987 study, Rogers developed an innovative analytical approach that placed calves in sterile conditions and helped scientists understand animals’ susceptibility to, and the pathology of, the disease. Rogers retired in 2014.
When Loy arrived at Nebraska in 2012 and learned of these longstanding connections to study of the disease, “I thought this would be a really fitting area for us to continue on in our legacy.”
In preparing to share bacterial strains with ARS for their recent cooperative study of bovine pinkeye, Loy understood the fuller significance when he took out a particular sample from freezer storage.
It was the same bacterial strain Pugh and Rogers had used decades ago for their study.
And so the chain of scientific inquiry continues, across the generations.
Share
News Release Contact(s)
Related Links
Tags
High Resolution Photos

HIGH RESOLUTION PHOTOS
HIGH RESOLUTION PHOTOS








