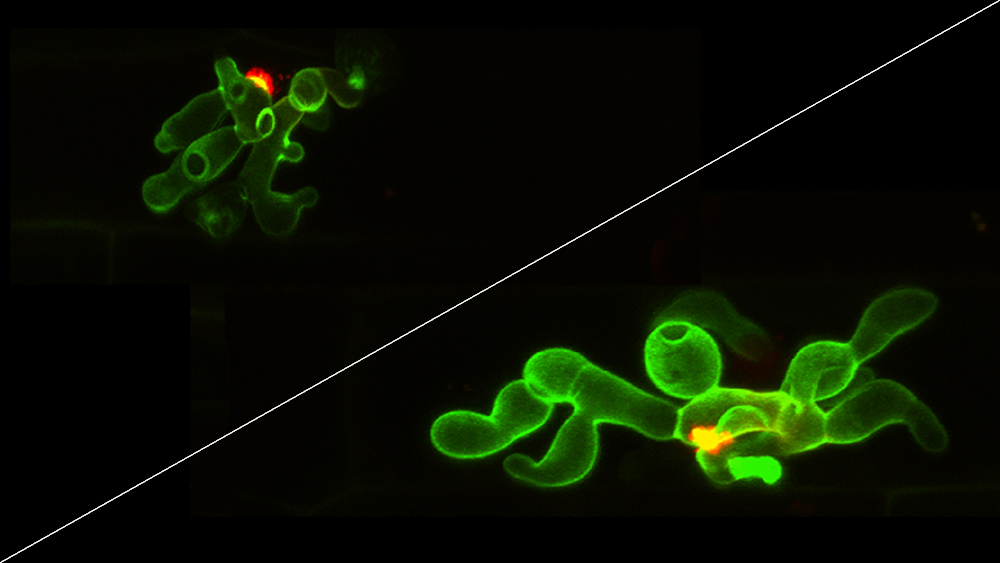
New research from the University of Nebraska-Lincoln has revealed how the fungus behind a destructive rice disease evades the plant’s first immune response and silences the molecular sirens that mobilize reinforcements.
The team’s identification of a key fungal gene and associated protein could inform genetic modification efforts to combat rice blast disease, which annually spoils between 10 and 30 percent of rice yields – enough to feed as many as 60 million people worldwide. Recent disease outbreaks caused by the fungus have ranged from Brazil to Bangladesh.

When a rice plant recognizes a signature component in the cell walls of invading fungi or picks up on other signs of intrusion, it responds by unleashing a burst of reactive oxygen species – molecules that easily react in the cell and become toxic in large doses. In addition to serving as a scorched-earth strategy that sacrifices some cells to neutralize an invasion, the burst alerts the rest of the plant to the threat and cues secondary defenses.
But the Nebraska team found that a protein called Nmo2 helps the Magnaporthe oryzae fungus catalyze chemical reactions that enable it to feed on nitrogen-based molecules and suppress the damage from reactive oxygen species. In doing so, the authors said, the fungus avoids detection long enough to build up its forces in living rice cells before spreading to and destroying others throughout the rice plant.
Marroquín-Guzman, Richard Wilson and their colleagues concluded that the NMO2 gene also supports the deployment of so-called effector proteins, which assist infection by intercepting the distress calls sent out by plant cells. Like other plants, rice has evolved genes to recognize the telltales of effector-related damage and coordinate a counterattack.

Yet the team discovered that the initial burst of reactive oxygen species is, by itself, enough to disrupt the accumulation of effectors and ultimately stymie the infection if not suppressed by the fungus. Though the researchers don’t yet know why this is the case, they said the finding could redirect existing efforts or stimulate new approaches to fighting rice blast disease.

“That would conceivably enable you to develop more general approaches to breeding, whereas at the moment, you’re mostly relying on deploying rice with specific blast-resistance genes.”
Wilson said he could envision editing rice genes that promote the burst of reactive oxygen species, perhaps by cranking up their sensitivity to ensure that the burst occurs before the fungus can suppress it.
The adaptability of the M. oryzae fungus, which relies on effector mutations to overcome resistance every few growing cycles, makes that potential especially relevant. As it stands, Wilson said, the fungus can actually overcome resistance “before a new rice variety even gets to the field, because sometimes the development can take 10 years or so.”
“All of these things that we’re talking about are new avenues,” he said. “Some of them may turn out to be profitable, some may be dead ends, but we’re excited about the prospects.”
The team’s study appeared in the journal Nature Microbiology. Marroquín-Guzman and Wilson authored the paper with Christian Elowsky, assistant professor of practice in agronomy and horticulture; Janet Wright, research technologist with the Department of Plant Pathology; David Hartline, a former undergraduate research assistant in Wilson’s lab; and Creighton University’s Travis Bourret.
The researchers received support from the National Science Foundation and the U.S. Department of Agriculture’s National Institute of Food and Agriculture.