New research led by the University of Nebraska-Lincoln has provided the first direct evidence that an algae-infecting virus can invade and potentially replicate within some mammalian cells.
Known as Acanthocystis turfacea chlorella virus 1, or ATCV-1, the pathogen is among a class of chloroviruses long believed to take up residence only in green algae. That thinking changed with a 2014 study from Johns Hopkins University and UNL that found gene sequences resembling those of ATCV-1 in throat swabs of human participants.
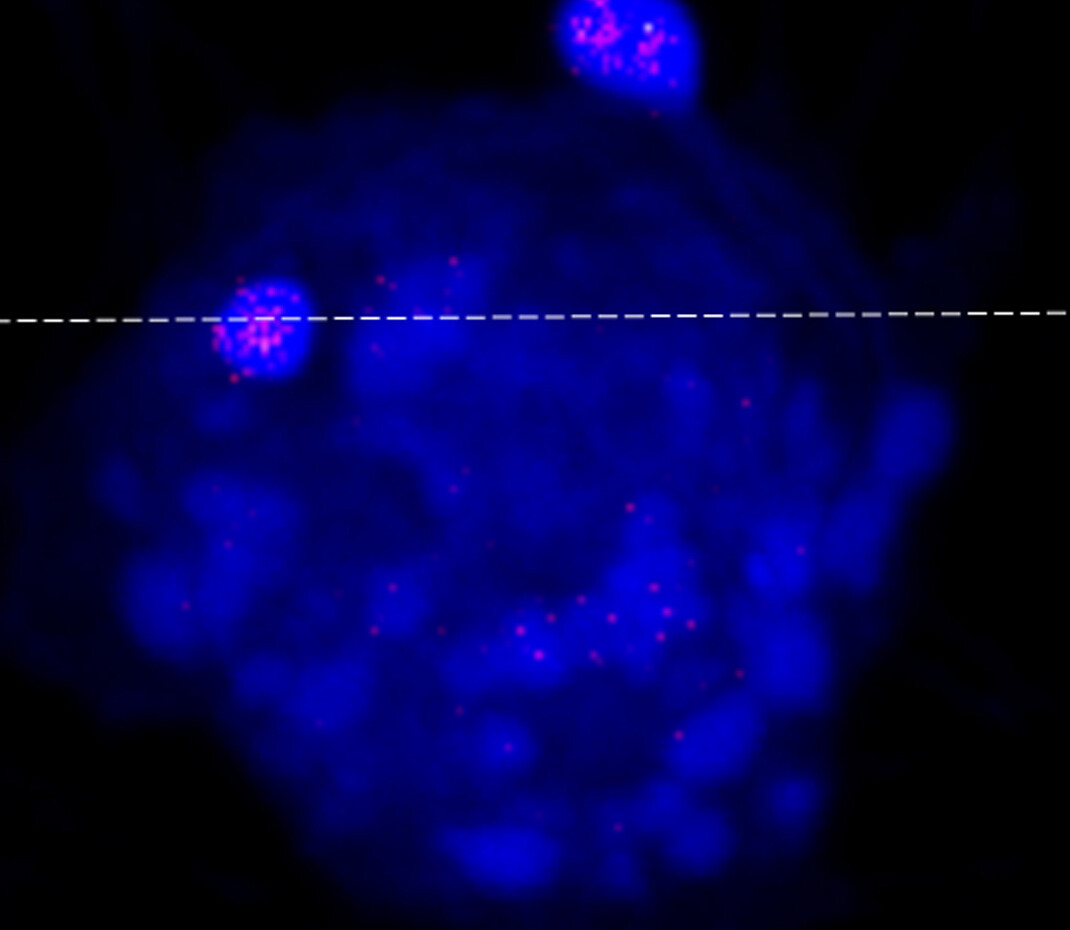
The new study, published in the Journal of Virology, introduced ATCV-1 to macrophage cells that serve critical functions in the immune responses of mice, humans and other mammals. By tagging the virus with fluorescent dye and assembling three-dimensional images of mouse cells, the authors determined that ATCV-1 successfully infiltrated them.
The authors also measured a three-fold increase in ATCV-1 within 24 hours of introducing the virus. The relatively modest spike nevertheless suggests that ATCV-1 can replicate within the macrophage cells, according to co-author David Dunigan.
Though a few studies have documented viruses jumping from one biological kingdom to another, chloroviruses were previously thought to have a limited “host range” that stopped well short of the animal kingdom, Dunigan said.
“A few years ago, no one I know would have made a prediction like this,” said Dunigan, research professor of plant pathology and member of the Nebraska Center for Virology. “You probably would’ve been laughed out of the room. But we are now in the middle of something that is so very interesting.”
The macrophage cells underwent multiple changes characteristic of those breached by a virus, Dunigan said. These changes eventually included a form of programmed death that virologists consider an innate “scorched earth” defense against the spread of viruses, which require living cells to survive and replicate.
Before dying, the cells exhibited multiple signs of stress that tentatively support links to mild cognitive impairments first reported in the 2014 paper. The new study measured a post-viral rise in interleukin 6, a cellular protein that previous research has linked with diminished spatial learning and certain neurological diseases. The authors also reported an increase in nitric oxide, an important signaling molecule that has been associated with memory impairments when produced in excess.
The 2014 investigation, which was initially designed to test the cognitive functioning of human participants, found that those with the ATCV-1 DNA performed slightly worse on measures of visual processing and visual motor speed. Mice inoculated with the virus showed similar deficits in memory and attention while navigating mazes. The 2014 paper further suggested that ATCV-1 altered the expression of more than 1,000 genes in the rodent hippocampus, an area of the brain tied to memory and spatial navigation.
The new study’s authors are continuing their collaboration with Johns Hopkins in the hope of ultimately confirming whether and how the virus contributes to any cognitive deficits suggested by the initial studies.
“It is still unclear whether the factors induced by the cell-based virus challenge could also be induced in the whole animal, and whether the induced factors cause cognitive impairments in the animal or the human,” said co-author Tom Petro, professor of microbiology and immunology at the University of Nebraska Medical Center.
Dunigan said he and his colleagues are also searching for other cellular responses to ATCV-1 while investigating how these responses might drive systemic changes in mice.
“These are pretty big, unexplored questions,” Dunigan said. “There are so many very basic virological questions that we can and want to ask.”
The study was co-authored by James Van Etten, a William Allington Distinguished Professor of plant pathology; Irina Agarkova, research assistant professor of plant pathology; You Zhou, research professor at the Morrison Microscopy Core Research Facility of the Center for Biotechnology; and Robert Yolken, director of the Stanley Neurovirology Laboratory at Johns Hopkins University.
The team’s research was supported in part by the National Center for Research Resources, part of the National Institutes of Health, under grant number P30-RR031151.